Disposable Infusion Set /I. V Set with Ce, ISO, GMP
Model NO.: infusion set
Logo Printing: With Logo Printing
Medical Device Regulatory Type: Type 3
Medical Devices Reg./Record No.: Gxzz20153661379
Brand: Jmd or OEM
Certificate: CE, ISO, GMP
ISO Certificate: ISO13485, ISO9001
Tubing Length: 1.5m or OEM
Raw Material: Can Be Dehp Free
Trademark: JMD or OEM
Transport Package: Blister or PE Packing
Specification: ISO, CE
Origin: China
HS Code: 90183900
Model NO.: infusion set
Logo Printing: With Logo Printing
Medical Device Regulatory Type: Type 3
Medical Devices Reg./Record No.: Gxzz20153661379
Brand: Jmd or OEM
Certificate: CE, ISO, GMP
ISO Certificate: ISO13485, ISO9001
Tubing Length: 1.5m or OEM
Raw Material: Can Be Dehp Free
Trademark: JMD or OEM
Transport Package: Blister or PE Packing
Specification: ISO, CE
Origin: China
HS Code: 90183900
PRODUCT SPECIFICATION



Â
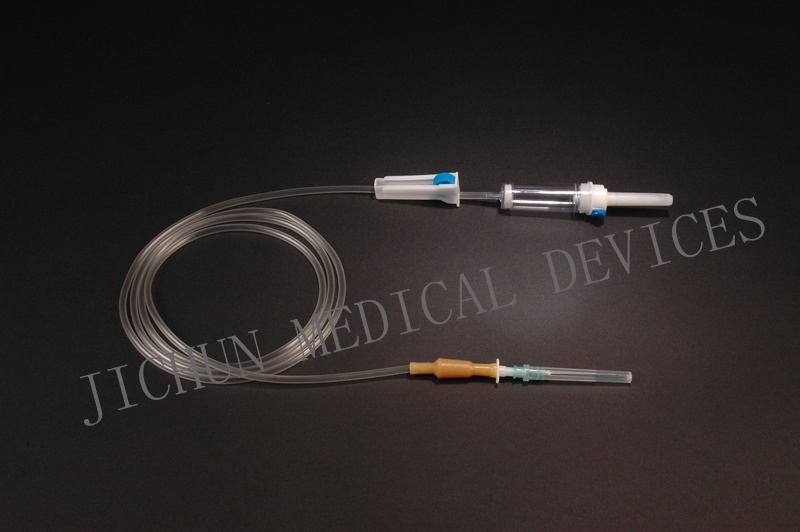
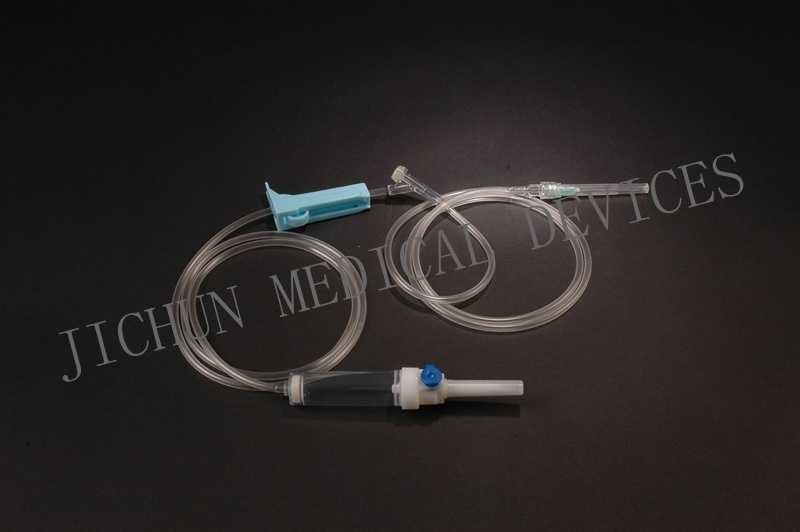
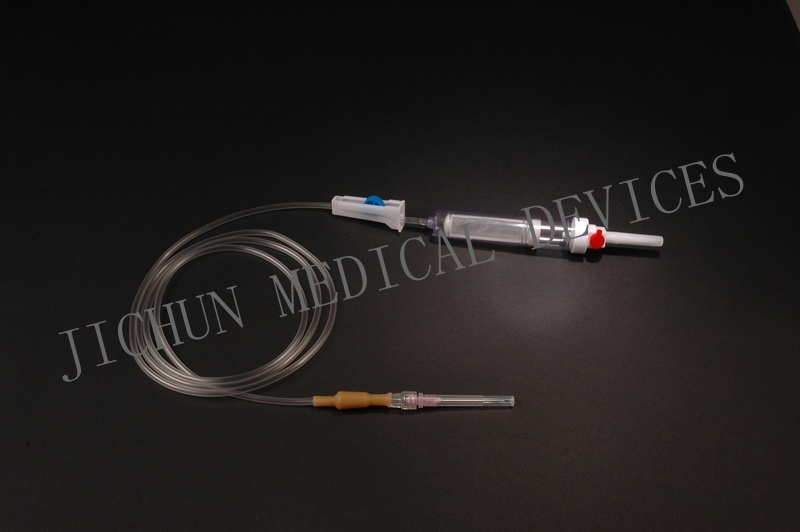
| Type | infusion set |
| Material | PVC |
| Certification | CE, SGS ,ISO13485 |
| Sterilization | Ethylene Oxide |
| Quality Guarantee Period | 3 years |
| Package | PE or Blister |
| Specification | CE , ISO , GMP |
| Tube material | PVC/Non- PVC |
| Feature | Disposable |
| Needle | with and without needle |
| Stype | Luer Slip or Luer lock |
| Tube length | 1.5m or OEMÂ |
| Components | Plastic Spike |
| Air vent | With / without air vent |
| Filter | with / without filter |



Â



FEATURES
1.infusion sets--with CE ISO manufacturer
2, 50% products are for eur, midest and Africa markets
3, annual qty to 0.1billion PCS
Infusion sets
4.Main accessories: Vented spike, drip chamber, fluid filter, flow regulator, latex tube, luer lock connector.
Protective cap for closure piercing device made of polyethylene with internal thread that prevents the bacteria from coming in, but allows the entrance of eto gas. Apporoximately 15 drops/ml, 20 drops/ml
5.Closure piercing device made of white PVC, with sizes according to ISO 1135-4 standards. Apporoximately 15 drops/ml, 20 drops/ml
6.Drip chamber made of soft PVC, sizes according to the ISO 8536-4 standards.
7.Flow regulator made of polyethylene.
8.Soft and kink resistant medical grade PVC tubing.
9.Terminal fitting protective cap (luer slip or luer-lock adapter upon request) made of PVC or polystyrene, 10.according to the ISO 594/1 and 594/2 standards.
11.Terminal fitting protective cap made of polyethylene.
12.Options available:
- with or without air vented spike;
- with or without needle;
- with or without "y" injection port;
- luer lock or luer slip connector;
| product |  components |  MOQ | packing |
| Disposable Sterile Latex Tube Infusion Set | drip chamber with wings, tubing, flow regulator with wings, lates tube, luer lock connector | Â 100,000pcs | Â PE, Blister |
About us
Jiangsu Jichun Medical Devices Co., Ltd. was established in 1988 (with the original name Zhenglu Medical Materials Factory), is located in fertile Yangtze River Delta of Changzhou City, with the provincial road 232 passing by the gate of the company, with convenient transportation and advantageous environment. In 2008 in order to enlarge production scale, the company began land acquisition in Zhenglu industrial development zone and built the new plants, now covers a total area of 36, 000 square meters, with building area of 18, 000 square meters and class 100, 000 cleaning workshop area of 5, 500 square meters.
The company mainly deals with the development, production and marketing of the medical polymer medical devices for single use. The production capacity of the main products is: 1, 000 million sets of injection needles per year, 600 million sets of syringes per year and 100 million sets of infusion devices per year. The company is with beautiful surroundings, advanced equipments, well-equipped facilities, and all kinds of advanced product measuring instruments and scientific test methods. The "Jichun" brand products produced in the company sell well worldwide, to Europe, America and Southeast Asia. Customers have high opinion of the quality and the service, with the cooperating partners and sales volum continuously increasing.
The company has a period of more than twenty years development history, with the distinctive human and geographic advantages that Changzhou is the old empire city and the location in Shanghai and Nanjing special economic zone, develops its own specialties in the aspects of injection molding, automatic assembly and packaging. The company has engaged senior top management, recruited lots of technicians, and established a complete set of quality management system. According to the requirements of ISO 13485: 2003/YY/T0287-2003 Medical devices_Quality management system_Requirements for Regulatory Purposes, Medical devices good manufacturing practice (try out), Medical devices good manufacturing practice Sterile medical devices implementation principles and inspection and evaluation standards (try out), US21 CFR Part 820 Medical devices current good manufacturing practice (cGMP), RDC59 Medical devices good manufacturing practice (GMP) and MDD 93/42/EEC Medical devices directive, assure the implementation and operation of the quality management system in the company.
In 2001 the company was appraised "credible production of quality labeled enterprise" by Chinese Association for Medical Devices Industry, Chinese Nursing Association and Chinese Fundation for Customer Protection. In 2002 the company passed ISO9002 and CE Certification, and in 2004, passed the ISO9001/ISO13485 and CE Certification. We have pioneered a developing road dominant by the precise manufacture of medical devices for single use.
In the new century, Jichun people will base on the market, look to the future, keep striving and forge ahead. We will seek the development of the company by the innovation of the concept, technology and system, to contribute to the human health.
Â
PRODUCT SPECIFICATION



Â



| Type | infusion set |
| Material | PVC |
| Certification | CE, SGS ,ISO13485 |
| Sterilization | Ethylene Oxide |
| Quality Guarantee Period | 3 years |
| Package | PE or Blister |
| Specification | CE , ISO , GMP |
| Tube material | PVC/Non- PVC |
| Feature | Disposable |
| Needle | with and without needle |
| Stype | Luer Slip or Luer lock |
| Tube length | 1.5m or OEMÂ |
| Components | Plastic Spike |
| Air vent | With / without air vent |
| Filter | with / without filter |



Â



FEATURES
1.infusion sets--with CE ISO manufacturer
2, 50% products are for eur, midest and Africa markets
3, annual qty to 0.1billion PCS
Infusion sets
4.Main accessories: Vented spike, drip chamber, fluid filter, flow regulator, latex tube, luer lock connector.
Protective cap for closure piercing device made of polyethylene with internal thread that prevents the bacteria from coming in, but allows the entrance of eto gas. Apporoximately 15 drops/ml, 20 drops/ml
5.Closure piercing device made of white PVC, with sizes according to ISO 1135-4 standards. Apporoximately 15 drops/ml, 20 drops/ml
6.Drip chamber made of soft PVC, sizes according to the ISO 8536-4 standards.
7.Flow regulator made of polyethylene.
8.Soft and kink resistant medical grade PVC tubing.
9.Terminal fitting protective cap (luer slip or luer-lock adapter upon request) made of PVC or polystyrene, 10.according to the ISO 594/1 and 594/2 standards.
11.Terminal fitting protective cap made of polyethylene.
12.Options available:
- with or without air vented spike;
- with or without needle;
- with or without "y" injection port;
- luer lock or luer slip connector;
| product |  components |  MOQ | packing |
| Disposable Sterile Latex Tube Infusion Set | drip chamber with wings, tubing, flow regulator with wings, lates tube, luer lock connector | Â 100,000pcs | Â PE, Blister |
About us
Jiangsu Jichun Medical Devices Co., Ltd. was established in 1988 (with the original name Zhenglu Medical Materials Factory), is located in fertile Yangtze River Delta of Changzhou City, with the provincial road 232 passing by the gate of the company, with convenient transportation and advantageous environment. In 2008 in order to enlarge production scale, the company began land acquisition in Zhenglu industrial development zone and built the new plants, now covers a total area of 36, 000 square meters, with building area of 18, 000 square meters and class 100, 000 cleaning workshop area of 5, 500 square meters.
The company mainly deals with the development, production and marketing of the medical polymer medical devices for single use. The production capacity of the main products is: 1, 000 million sets of injection needles per year, 600 million sets of syringes per year and 100 million sets of infusion devices per year. The company is with beautiful surroundings, advanced equipments, well-equipped facilities, and all kinds of advanced product measuring instruments and scientific test methods. The "Jichun" brand products produced in the company sell well worldwide, to Europe, America and Southeast Asia. Customers have high opinion of the quality and the service, with the cooperating partners and sales volum continuously increasing.
The company has a period of more than twenty years development history, with the distinctive human and geographic advantages that Changzhou is the old empire city and the location in Shanghai and Nanjing special economic zone, develops its own specialties in the aspects of injection molding, automatic assembly and packaging. The company has engaged senior top management, recruited lots of technicians, and established a complete set of quality management system. According to the requirements of ISO 13485: 2003/YY/T0287-2003 Medical devices_Quality management system_Requirements for Regulatory Purposes, Medical devices good manufacturing practice (try out), Medical devices good manufacturing practice Sterile medical devices implementation principles and inspection and evaluation standards (try out), US21 CFR Part 820 Medical devices current good manufacturing practice (cGMP), RDC59 Medical devices good manufacturing practice (GMP) and MDD 93/42/EEC Medical devices directive, assure the implementation and operation of the quality management system in the company.
In 2001 the company was appraised "credible production of quality labeled enterprise" by Chinese Association for Medical Devices Industry, Chinese Nursing Association and Chinese Fundation for Customer Protection. In 2002 the company passed ISO9002 and CE Certification, and in 2004, passed the ISO9001/ISO13485 and CE Certification. We have pioneered a developing road dominant by the precise manufacture of medical devices for single use.
In the new century, Jichun people will base on the market, look to the future, keep striving and forge ahead. We will seek the development of the company by the innovation of the concept, technology and system, to contribute to the human health.
Â
Mg-Sc Alloy,Aluminium Scandium Alloy,Al-Sc2 Alloy
Polyurethane Catalyst,Rubber Auxiliary Co., Ltd. , http://www.wflonggang.com