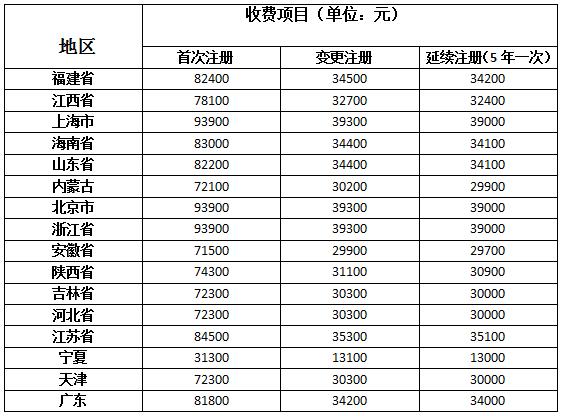
Guangdong Province issued medical equipment registration fee standard: the first single registration of 81,800 yuan
Recently, Guangdong Province announced the registration fees within the second category of medical devices, the standard registration fee for the first time within the second category of medical products to 81,800 yuan fee, change registration fee 34,200 yuan, 34,000 yuan continued registration fee. At the same time, if the "Administrative Measures for the Registration of Medical Devices" and the "Administrative Measures for the Registration of In Vitro Diagnostic Reagents" are subject to change of registration items, no registration fee will be charged. Small and micro enterprises that meet the "SME Standardization Regulations for SMEs" (Ministry of Industry and Information Technology (2011) No. 300) apply for registration of innovative medical device products, and are exempt from the initial registration fee. The certification standards for innovative medical device products shall be implemented in accordance with the relevant provisions of the State Food and Drug Administration. The fee standard shall be officially implemented on March 1, 2017.

Up to now, 16 provinces and municipalities across the country have issued registration fees for the second category of medical device products in China, of which Ningxia has the lowest charging standard.
We are a professional Chinese manufacturer of Preservatives Agent;we supply various products of Fresh Keeping Agent, and can providing product images and basic parameters with each Preservatives Agent. We have the perfect after-sales service and technical support. Please do not hesitate to contact us, Look forward to your cooperation!
Potassium Sorbate,Fresh Keeping Agent,Sodium Benzoate,Benzoic Acid,Calcium Propionate
Xi'an Quanao Biotech Co., Ltd. , https://www.quanaobiotech.com