The latest solution: a fully automated single-cell injection technique with mechanical sensing
Fully automatic single-cell injection technology with mechanical sensing - the latest solution for in vivo fully automated single-cell injection
Since the advent of the micro-cell injection in the 1970s, it has become an important means for proteins, nucleic acids, peptides, drugs, and microparticles to enter cells. However, this technique is too demanding for the operator, and the microinjection needle for injection is difficult to prepare and becomes a technical barrier that is difficult to be widely applied. Nowadays, the emergence of FluidFM technology has overcome this technical barrier.
FluidFM is the product of the perfect combination of AFM and microfluidic technology
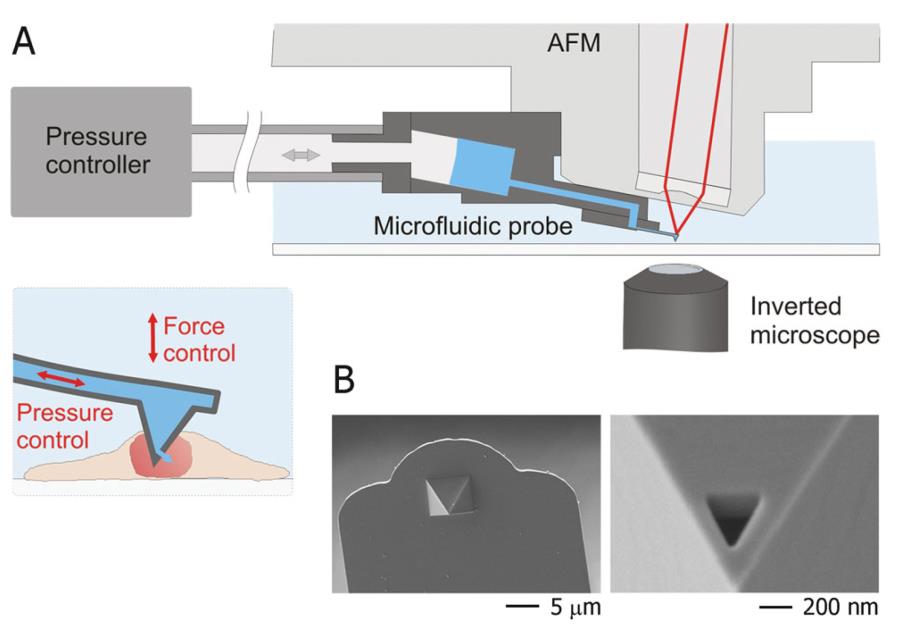
The latest nanotechnology has given a new turn to microfluidic injection technology. The hollow nanoprobes manufactured by the nanotechnology technology enable the probe to have the ability to inject into the detection object while performing surface mechanical detection. This feature allows the probe to sense cell surface stress, giving the user the most direct sense of the current state of the probe, helping the user to quickly understand the current state of the probe and determine the timing of the injection. The perfect combination with microfluidic control also gives perfect control of injection accuracy. In addition, the nanoprobe used is very small, and the head of the Syringe is only 400 nm, which causes almost no damage to cells.
Dynamic demonstration of fully automated, highly efficient U2OS cell injection
How does FluidFM work?
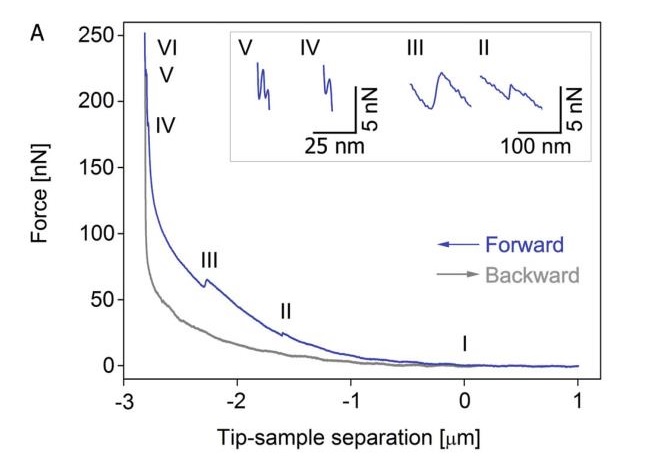
Mechanical changes occur when the syringe pierces the cell membrane or the nuclear membrane. The FluidFM system with mechanical detection can detect this mechanical change in the force spectrum.
As shown in Figure A, the mechanical curve of (I) begins to rise, indicating that the probe is in contact with the cell surface. (II) When the syringe punctures the cell membrane, the resistance of the probe surface is immediately lowered. So we can observe the first relaxation peak in the graph. (III) Then we will observe the second relaxation peak when the probe continues to deepen and the core is released and the nuclear membrane is pierced. (IV) As the probe continues to move down and begin to approach the slide, the pressure begins to increase rapidly until the nuclear membrane is punctured. After (V), as the probe continues to deepen, the cell membrane is also penetrated. (VI) At this time, the probe is in full contact with the glass, and the force increases as the Z-axis moves downward.
FluidFM's injection accuracy is extremely high
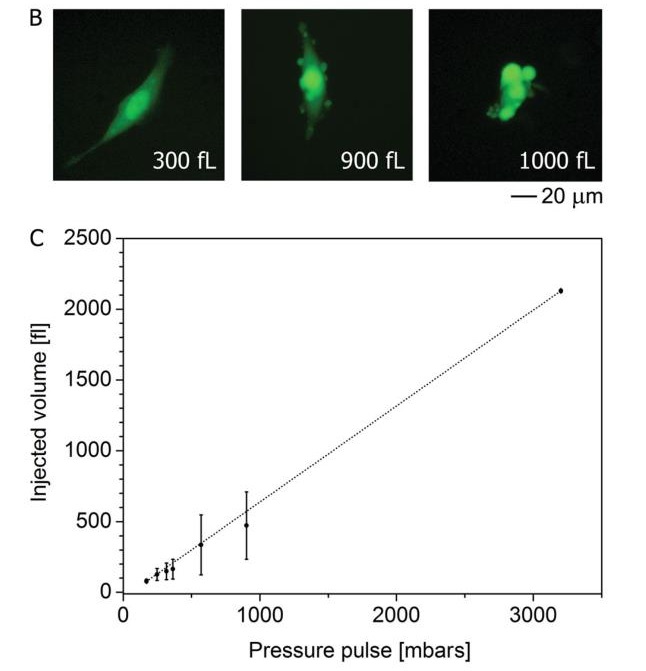
Cell injection using FluidFM technology is a fast and reliable means. First, the amount of single cell injection is considered as shown in b. No changes in cell morphology were observed when 800 fL (corresponding to 20% of the total cell volume) was injected. After the injection volume reached 900 fL, vesicles began to appear on the cell surface. Further, increasing the injection volume, the cell morphology changes. The injection volume of more than 30 cells was then counted for repeated injections and injections (Table C). From the results, when the pressure injection is less than 500 mbar, the injection volume is linear, the stability is excellent, and the reproducibility is good.
Injection with FluidFM does not affect cell viability
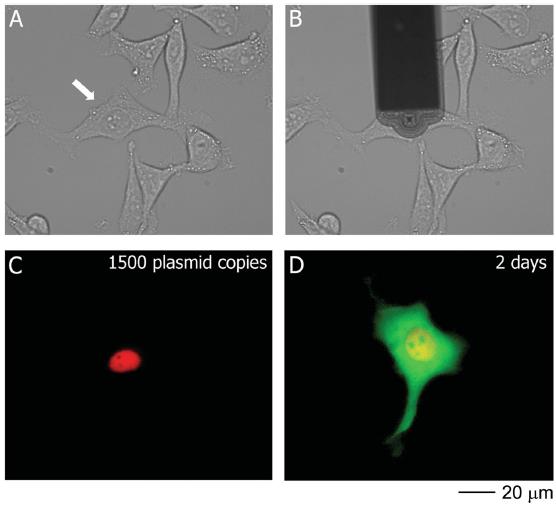
Subsequently, in order to investigate the effect of FluidFM on cell viability, the researchers chose to mix pmaxGFP and RFP fluorescent dye into the nucleus. Since the injection contains RFP dye, the injection site can be monitored immediately. The results showed that FluidFM accurately transferred the dye and plasmid into the nucleus without any dye flowing into the cytosol. The expression of GFP fluorescent protein was then observed at two hours of observation and peaked after 17 hours.
Summarizing simple operation and precise injection, FluidFM technology is unique in the field of microinjection. This powerful tool will surely shine in the regular and special injection fields.
Swiss Cytosurge multi-function single-cell microscopic operating system FluidFM BOT
——Injection, extraction, sorting and integration of single cell manipulation solutions

The multi-function single-cell microscopic operating system—FluidFM BOT is a single-cell operating system that integrates an atomic force system, a microfluidic system, and a cell culture system. The main functions include single cell injection, single cell extraction, and single cell separation.
FluidFM BOT greatly facilitates single-cell level studies, especially for precision medicine, single-cell biology, single-cell mass spectrometry, single-cell gene editing, and drug discovery.
references
[1] C. Mueller, A. Graessmann, M. Graessmann, Trends Biochem. Sci1980, 5, 60 .
[2] Y. Zhang, L.-C. Yu, Curr. Opin. Biotechnol. 2008 , 19 , 506 .
[3] P. Candeloro, L. Tirinato, N. Malara, A. Fregola, E. Casals, V. Puntes, G. Perozziello, F. Gentile, ML Coluccio, G. Das, C. Liberale, F. De Angelis , E.
Di Fabrizio , Analyst 2011 , 136 , 4402 .
[4] A. Dorr, V. Kiermer, A. Pedal, H.-R. Rackwitz, P. Henklein, U. Schubert, M.-M. Zhou, E. Verdin, M. Ott, EMBO J. 2002, 21,2715 .
[5] M. Rechsteiner , L. Kuehl , Cell 1979 , 16 , 901 .
[6] C. Lappe-Siefke, C. Maas, M. Kneussel, J. Neurosci. Methods2008, 175, 88
[7] WW Franke , E. Schmid , S. Mittnacht , C. Grund , J. Jorcano , Cell1984 , 36 , 813 .
[8] RB Silver, Dev. Biol. 1989, 131, 11 .
[9] MP King , G. Attardi , Cell 1988 , 52 , 811 .
[10] MR Capecchi , Cell 1980 , 22 , 479 .
[11] CM Cuerrier, R. Lebel, M. Grandbois, Biochem. Biophys. Res.Commun. 2007, 355, 632 .
[12] O. Loh, R. Lam, M. Chen, N. Moldovan, H. Huang, D. Ho, HD Espinosa, Small 2009, 5, 1667 .
[13] K. Yum , N. Wang , M.-F. Yu , Nanoscale 2010 , 2 , 363 .
[14] S. Han, C. Nakamura, I. Obataya, N. Nakamura, J. Miyake, Biochem. Biophys. Res. Commun. 2005, 332, 633 .
[15] S.-W. Han, C. Nakamura, N. Kotobuki, I. Obataya, H. Ohgushi, T. Nagamune, J. Miyake, Nanomed. Nanotech. Biol. Med. 2008, 4,215 .
[16] GS Michael , MF Erica , LZ Barry , HB Haim , Nanotechnology 2008 , 19 , 015101 .
[17] X. Chen, A. Kis, A. Zettl, CR Bertozzi, Proc. Natl. Acad. Sci. USA2007, 104, 8218 .
[18] A. Meister, M. Gabi, P. Behr, P. Studer, J. Vörös, P. Niedermann, J. Bitterli, J. Polesel-Maris, M. Liley, H. Heinzelmann, T. Zambelli, Nano Lett. 2009, 9 ,
2501 – 2507 .
[19] M. Yokokawa, K. Takeyasu, SH Yoshimura, J. Microsc. 2008, 232, 82.
[20] G. Minaschek, J. Bereiterhahn, G. Bertholdt, Exp. Cell Res. 1989, 183, 434.
[21] GM Lee , J. Cell Sci. 1989 , 94 , 443 .
[22] D. Schindelhauer , A. Laner , Gene Ther. 2002 , 9 , 727 .
 |  |
 |  |
Ningbo Siny Medical Technology Co., Ltd , https://www.sinymedical.com