They all say that they want to "fill iron". Do you really need iron?
Iron is an essential trace element in the human body. It acts as a component of an enzyme or protein and participates in many physiological processes such as oxygen transport, cell proliferation and energy metabolism in the body. Iron is good, but it is also an invisible double-edged sword in the body. A large amount will lead to a series of diseases, such as iron deficiency anemia and hemochromatosis.
Therefore, the human body has a system to regulate iron balance, which is essential for effective prevention and control of disease development.
Iron is an important raw material for human hematopoietic red blood cells. Unfortunately, the human body cannot synthesize iron. The main source of iron in the body is the recycling of red blood cells with a life span of only 120 days. Hundreds of millions of aging red blood cells are swallowed by spleen macrophages (an immune cell with phagocytic function), and then released through a series of degradation processes, and temporarily stored in the spleen for a new one. The production of red blood cells is required to maintain the normal number of red blood cells in the body.
In contrast, normal people get very little iron from food through the digestive tract (mainly the duodenum) every day. Therefore, blind iron supplementation does not mean that the body can absorb a lot of iron.
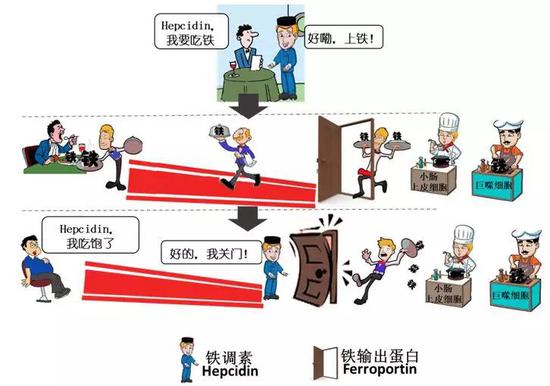
"Invisible hand " - hepcidin
So, which molecules are involved in regulating the balance of supply and demand for iron? It has been scientifically discovered that this relies mainly on a polypeptide called Hepcidin secreted by the liver. It acts like an invisible "hand" to shut down the only iron output "valve" in the body, Ferroportin.
When the body prompts iron deficiency, this "hand" will not close the "valve", so that the iron is continuously released from the spleen to the blood and transported to the iron part such as bone marrow, and the iron absorbed by the duodenum will also It increases to a certain extent; when the body prompts too much iron, the "hand" will immediately turn off the "valve", thereby effectively reducing the iron excretion from the spleen and the absorption of iron by the duodenum.
Environmental or genetic factors will lead to disorders of iron metabolism, and further trigger anemia, iron load and other diseases, including thalassemia and hemochromatosis, which is a typical iron-loaded disease.
The thalassemia is named after it is mostly found in the Mediterranean region, and it is also common in southern China. The patient is ruptured when the red blood cells mature due to genetic defects. The body is seriously lacking normal red blood cells that transport oxygen. Under the regulation of the feedback signal, the body is forced to produce more red blood cells. This process stimulates the intake of a large amount of iron, but the newly manufactured red blood cells still cannot. Normal maturity. This vicious cycle causes more and more ruptured red blood cells that are engulfed by macrophages, eventually causing iron to accumulate in the liver, spleen and serum.
It can be seen that anemia does not mean that there is a certain iron deficiency in the body. Hemochromatosis is also caused by a genetic defect that causes an "invisible hand" to fail, which in turn causes iron to deposit in the body and darken the skin.
At present, the treatment of iron-loaded diseases mainly uses iron, bloodletting and splenectomy. However, these treatments have many limitations, which seriously affect the therapeutic effect and urgently need to find new drugs. Studies on the pathogenesis have found that iron deposits are mainly due to the small number of "hands" that are not sufficient to shut down all "valves." Therefore, increasing the amount of hepcidin - these "hands" is considered to be a potential therapeutic target for effective iron load reduction.
Recently, the team of researchers Liu Sijin of the State Key Laboratory of Environmental Chemistry and Ecotoxicology of the Research Center for Eco-Environmental Sciences of the Chinese Academy of Sciences has made many progress in the study of metal metabolism abnormalities and toxicity.
- They found more activators that effectively stimulate the expression of hepcidin
The team worked with a number of domestic and foreign organizations to find activators of hepcidin. Through the screening of 210 thiazolidinone compounds, three activators were found to stimulate the expression of hepcidin ("hand"). Through the mouse model of hepcidin gene deletion, it was confirmed that the target of these three compounds is the expression of hepatic Hepcidin. Using hemochromatosis (Hfe-/-) mice as a model, it was confirmed that these compounds can significantly reduce the iron load level in the liver of mice.
At the same time, it was also confirmed in the mouse model of thalassemia (Hbbth3/+) that these compounds can both reduce the iron load in tissues in mice and improve the anemia problem in mice. Mechanistic studies have confirmed that these compounds stimulate the expression of hepcidin in the liver mainly by inhibiting two protein molecules called TMPRSS6 and P-ERK (Fig. 1). Related research results were published in Haematologica.
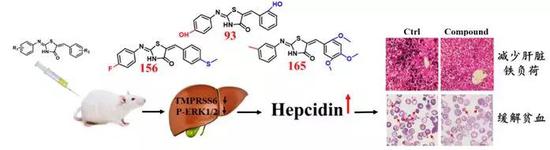
Figure 1. Thiazolone compounds prevent iron load and improve anemia by increasing the expression of hepcidin (Hepcidin)
- They found the distribution of iron in the liver
At present, little is known about the characteristics and extent of iron deposition in different regions of the liver under iron loading. The team found that iron in the liver was unevenly distributed, with high iron content in the vicinity of the portal vein and hepatic artery entrance; iron content was significantly reduced in the marginal region near the distal end of the liver (Fig. 2). Areas with more iron accumulation are more sensitive to changes in iron levels in the body. Mechanism studies have found that the difference in iron distribution in various regions of the liver is mainly determined by the pathway composed of hepcidin and iron export protein. Related research results are published in Advanced Science.

Figure 2 The cumulative distribution of iron in the liver shows significant regional differences
- They also discovered the expression pattern of hepcidin during pregnancy and synthesized a new type of iron chelator
In addition, the team also found changes in hepcidin in both normal and abnormal pregnancy, and confirmed that a new iron chelator, deferoxamine-caffeine dimer (DFCAF), has a higher Iron effect. Compared with deferoxamine alone (DFO), DFCAF is more cell permeable and can more efficiently sequester iron in cells, thereby inhibiting the growth and metastasis of tumor cells. More importantly, DFCAF significantly cleared tumor stem cells (CSCs) and reduced an important subset of cells (CD44+/high/CD24-/low and ALDH+/high). These findings provide new insights into the mechanisms of iron metabolism in normal and disease states. The research results are published in the American Journal of Hematology and Journal of Trace Elements in Medicine and Biology.
Source: Voice of the Chinese Academy of Sciences
Zhuhai Mingke Electronics Technology Co., Ltd , https://www.zhmkdz-electronics.com