MRI procedure for patients with cardiac implanted electronic devices

Figure 1: MRI check flow chart for patients with cardiac implanted electronic devices
MRI uses proton imaging to provide anatomical and functional images, which are popular because of their clear imaging and no radiation effects. They are also the first choice for brain tissue, low spinal cord, joints and other parts. However, some implantable electronic devices (CIEDs) such as pacemakers, implantable cardioverter defibrillators (ICDs), and more complex cardiac resynchronization treatment devices can affect imaging outcomes.
Therefore, these patients have had problems with MRI examinations for many years. Recently, Dr. Allon Eyal and others published a review of how to do MRI after heart implanted electronic devices in Circulation magazine, and answered questions for these questions.
What are heart electronic implant devices (CIEDs)?
The cardiac conduction system produces and conducts impulses, stimulating cardiomyocytes, causing contraction of cardiomyocytes. Heart rhythm disorders lead to abnormal heart function, which has serious consequences and even leads to death.
Cardiac electronic implant devices (CIEDs) monitor endogenous rhythms and can treat arrhythmias by transmitting electrical signals. Pacemakers can produce impulsive treatment of slow arrhythmias, and ICD can treat certain tachyarrhythmias.
How does MRI affect the function of cardiac electronic implant devices (CIEDs)?
The pacemaker and ICD are mainly composed of two parts. Part of it is the pulse generating device, which is usually placed in the patient
The upper left chest is subcutaneous. The second part is the lead, which connects the heart tissue to the pulse generator.
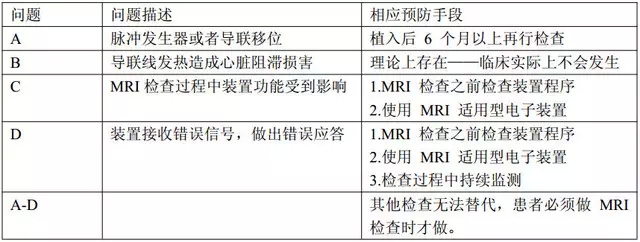
Both parts are affected by the electromagnetic force during the MRI examination, and the following problems occur: A magnetic force causes displacement of the pulse generator or lead; B causes conduction heat to damage the myocardium; C affects device function; D MRI check generates electrical signals Cardiac electronic implant devices (CIEDs) accept and respond incorrectly.
The management during the inspection process is shown in Table 1.
Table 1: Problems in the inspection and solutions
Advances in MRI examination in patients with cardiac electronic implant devices (CIEDs)
Despite various problems, some preclinical or clinical studies have shown that the impact of MRI on cardiac electronic implant devices (CIEDs) is exaggerated. Studies have shown that placement of cardiac electronic implant devices (CIEDs) for more than 6 months does not cause device displacement or tissue damage during MRI. Other studies have shown that cardiac electronic implant devices (CIEDs) are not affected as long as the device setup is well established during MRI and properly performed during the exam.
The following rules should be followed during and after the inspection:
1. Other tests are not a substitute for MRI and the electronics have been implanted for more than 6 months.
2. The function of the electronic device should be checked by a professional before the MRI examination, and the parameters should be adjusted according to the device and the patient's condition.
3. The electronic equipment should be continuously monitored during the MRI examination, and the cardiopulmonary resuscitation items should be prepared next to it.
4. Check the function of the electronic device immediately after the inspection, and review it one month later.
MRI suitable cardiac electronic implant device
The MRI-appropriate cardiac electronic implant device has been used clinically and is essentially contraindicated without MRI.
to sum up
Regardless of the type of cardiac electronic implant device, it should be carefully and properly performed during MRI examination. The specific recommended procedure can be seen in the opening flow chart.
Product Information:
Manufacturer supply sell high quality tryptamine CAS 61-54-1
Tryptamine CAS:61-54-1 is a monoamine alkaloid. It contains an indole ring structure, and isstructurally similar to the amino acid tryptophan, from which the name derives. Tryptamine
CAS:61-54-1 is found in trace amounts in the brains of mammals and is hypothesized to play a role as a neuromodulator or neurotransmitter.It is used in biological and pharmaceutical intermediates,with vasoactive, possibly neuromodulatory and other functions.
Name
Factory supply Dimethyl Tryptamine powder cas 61-54-1 with low price Tryptamine
Other name
1H-Indole-3-ethanamine;2-(1H-Indol-3-yl)ethanamine;3-(3-indolyl)ethylamine;4-2-Indol-3-yl-aethylamin;2-indol-3-ylethylamine;2-indol-3-yl-ethylamine;3-(2-aminoethyl)indole;
CAS
61-54-1
Molecular formula
C10H12N2
Molecular weight
160.22
Appearance
White or light yellow crystal
Purity
98%
Application
Organic intermediate;
Package
100g, 500g, 1Kg Aluminum plastic composite bag or 25kg cardboard barrel
Our company offers variety of products which can meet your multifarious demands.including API Powder.Pharmaceutical Intermediates.Vitamins Powder.Plant Extracts.Food Additive.Peptide Powder and so on We adhere to the management principles of "quality first, customer first and credit-based" since the establishment of the company and always do our best to satisfy potential needs of our customers. Our company is sincerely willing to cooperate with enterprises from all over the world in order to realize a win-win situation since the trend of economic globalization has developed with anirresistible force.
Product Photo:

|
Name |
Factory supply Dimethyl Tryptamine powder cas 61-54-1 with low price Tryptamine |
|
Other name |
1H-Indole-3-ethanamine;2-(1H-Indol-3-yl)ethanamine;3-(3-indolyl)ethylamine;4-2-Indol-3-yl-aethylamin;2-indol-3-ylethylamine;2-indol-3-yl-ethylamine;3-(2-aminoethyl)indole; |
|
CAS |
61-54-1 |
|
Molecular formula |
C10H12N2 |
|
Molecular weight |
160.22 |
|
Appearance |
White or light yellow crystal |
|
Purity |
98% |
|
Application |
Organic intermediate; |
|
Package |
100g, 500g, 1Kg Aluminum plastic composite bag or 25kg cardboard barrel |
Our company offers variety of products which can meet your multifarious demands.including API Powder.Pharmaceutical Intermediates.Vitamins Powder.Plant Extracts.Food Additive.Peptide Powder and so on We adhere to the management principles of "quality first, customer first and credit-based" since the establishment of the company and always do our best to satisfy potential needs of our customers. Our company is sincerely willing to cooperate with enterprises from all over the world in order to realize a win-win situation since the trend of economic globalization has developed with anirresistible force.
Product Photo:

Dmt Dimethyl Tryptamine,Nutrition Supplement Powder,Bulk Bcaa Powder,Bcaa 2:1:
Shaanxi YXchuang Biotechnology Co., Ltd , https://www.peptide-nootropic.com