Rigel's Fomatatinib treatment of kidney disease phase 2 clinical trial failed
Rigel's Fomatatinib treatment of kidney disease phase 2 clinical trial failed
April 04, 2018 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];On April 3, Rigel Pharmaceuticals, Inc. announced top-line data from a clinical phase 2 proof-of-concept trial of fostamatinib for IgA nephropathy (IgAN), a rare kidney autoimmune disease. The primary end point of the trial was the mean change in proteinuria in the fostamatinib dose group compared with the placebo control group in all study patients. The results of the study showed that the primary endpoint was not statistically significant. At the same time, in a pre-specified subgroup analysis of patients with baseline proteinuria over 1 g/day, preliminary data showed that patients treated with fostamatinib had more proteinuria compared with placebo (but this finding Nor is it statistically significant). Patients with proteinuria over 1 g/day have an increased risk of disease progression, and there is a large unmet clinical need for treatment in such patients. IgAN's current clinical trial guidelines recommend studying patients with proteinuria greater than 1 g/day at the time of trial enrollment. Further analysis, including histology, is expected to take place later this year.

Raul Rodriguez, President and CEO of Rigel Pharmaceuticals, said: "We found the results of the subgroup analysis to be encouraging, and patients and doctors have been challenging the management of the disease in the face of such serious illnesses without approved treatments. Prove the therapeutic potential of fostamatinib in patients with IgA nephropathy with high clinical need, ie the drug has potential benefits for patients with proteinuria over 1 g/day."
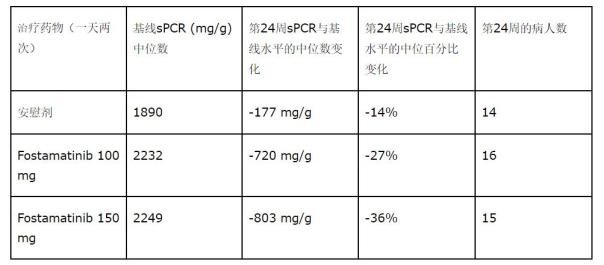
Fostamatinib is used in the treatment of patients with IgA nephropathy, and patients are recruited at multiple centers in the United States, Europe, and Asia. The patient was confirmed to be IgAN by biopsy and proteinuria over 500 mg/day. Patients (n=76) were randomized into three groups: placebo, Fostamatinib 100 mg twice daily, and Fostamatinib 150 mg twice daily for a 24-week period. This study evaluated the safety and efficacy of changes in proteinuria and renal function. The mean change in proteinuria (sPCR) was: placebo, 100 mg (twice a day), and 150 mg (twice a day) were -177, -577, -158 mg/g, respectively. Learning meaning.
Pre-specified subgroup analysis showed that patients receiving fostamatinib with baseline proteinuria greater than 1 g/day (sPCR>1000 mg/g) were dose-dependent at 24 weeks compared with placebo compared with placebo. Sexual proteinuria reduces the trend. In patients with baseline proteinuria greater than 2 grams, fostamatinib treatment showed a similar reduction in proteinuria compared to placebo.
Fostamatinib is well tolerated, with adverse reactions ranging from mild to moderate, with no new safety signs compared to the safety data for all indications for this drug. The most common adverse reactions are diarrhea, nausea, headache, high blood pressure and vomiting. Two patients in the 100 mg dose group and four patients in the 150 mg dose group discontinued the study due to adverse events. There were 6 patients with severe adverse reactions (SAEs), two patients in the placebo group, the 100 mg dose group, and the 150 mg dose group. Of the 6 patients, 1 in the fostamatinib group was drug-related SAE. One patient was fatal SAE but not related to the treatment.
Frederick Tam, a professor of medicine at Imperial College, said: "There are currently no special treatments for thousands of patients suffering from this serious disease. Therefore, there is a need to identify a potential method for treating IgA nephropathy to reduce progress. Risk of serious complications of kidney disease, the most serious complications may lead to dialysis and kidney transplantation. Rigel Pharmaceuticals and Imperial College's long-term cooperation is highly anticipated, data from this clinical phase 2 study showed fostamatinib within 6 months Reducing proteinuria levels in more advanced patients is a non-statistical trend, which is an important result because the risk of loss of renal function is higher when proteinuria increases and continues to be high."
Rigel plans to find a drug development partner to collaborate on follow-up clinical studies in IgAN patients. And the partner will be responsible for the commercialization of products outside fostamatinib in the United States.
Fostamatinib is a potent inhibitor of spleen tyrosine kinase, which inhibits BCR signaling and selectively inhibits the survival and proliferation of TCL1 leukemia cells in vivo, and can significantly prolong the survival of experimental animals. In addition, fostamatinib is active in both in vitro and in vivo models and has been used in phase II clinical studies of rheumatoid arthritis and immune thrombocytopenic purpura, as well as in relapsed and difficult to treat B-cell lung Hodgkin's lymphoma (NHL). ) and significant clinical activity in chronic lymphocytic leukemia. (Sina Pharmaceutical Compilation / Fan Dongdong)
Article Reference Source: Rigel Announces Topline Data from Proof-of-Concept Phase 2 Study of Fostamatinib in IgA Nephropathy
Walking aid, as its name implies, is a walking aid. Its function is to support weight, maintain standing balance, and assist walking, so as to realize compensation and improve walking ability.
As an assistive device, walking aids are indispensable in the rehabilitation training process of age, limb disability and limb injury, so how to choose a suitable walking aid is also a learning.
Walking aids are a large category, which can be divided into walking sticks, walking frames and wheelchairs from simple to complex.
What should we pay attention to when using?
1. Adjust the height of the walker according to the user's own conditions. The general principle is correct posture and comfortable use.
2. Necessary inspection shall not be less to ensure safe use.
â‘ Is there any problem with the deployment and storage of the walker; â‘¡ Check whether each part is loose and whether there is burr on the surface of walking aid; â‘¢ Whether the feet or wheels contact the ground smoothly and whether the wheels are flexible; â‘£ The locating pin is not firm, can it be fixed
3. Installation and removal of accessories under specific conditions
â‘ Provide cushion for rest; â‘¡ Sitting bath board for bathing; â‘¢ Toilet board for auxiliary toilet use; â‘£ Shelves and shopping bags for storing things. Use and carry according to the situation, grasp the measure, and the main purpose is to assist walking.
TRUTECH CO., LTD. is an enterprise specializing in the research, development, production and sales of professional OTC products.The main products include shower chairs, armrests, toilet racks and toilet armrests, bedside armrests, elderly chairs and sofas, crutches and walking aids, knee pads, waist supports and other equipment.
Walker,Hemiplegic Walker Bracke,Health Care Walker,Elderly Walker Medical Treatment
TRUTECH CO., LTD. , https://www.trutechealth.com