Characteristics and application of one-time stirring bioreactor
Effective tools for process development and control (quality attributes and critical process control)
In the past decade, disposable bioreactors have been widely accepted as an alternative to traditional stainless steel or glass bioreactors for pharmaceutical production and process development. In the biopharmaceutical industry, glass bioreactors are primarily used in process development and optimization, and as a scale-down model for process parameter validation. Therefore, whether it is a repetitive or one-time reactor, recreating the design characteristics of a production scale reactor is extremely important for achieving upstream purposes. 2-L, 5-L and 10-L working volume agitated bioreactors have gained wide acceptance and acceptance throughout the industry. Among them, 2-L volume reactor is the main equipment for process development. As a representative scale-down model, this type of reactor is easy to operate and has a large enough sampling volume.
Improve drug development efficiency
Timelines and workloads during process development can change significantly. It is also a challenge to maintain a sufficient number of experimental reactors throughout the process development cycle. Cleaning, installation, and autoclaving of glass containers requires additional time and effort from the experimenter, putting them into routine maintenance and not being able to perform other, more valuable work. For process development laboratories, the addition of disposable reactors can be interchanged with glass containers, providing significant flexibility during peak or maintenance periods, minimizing downtime for bioreactor controllers.
 Table 1 compares the glass and disposable reactors and it is expected that the controller life of the disposable bioreactor will increase by 25% over the glass reactor. If a disposable reactor is not used, the number of glass reactors needs to be increased to achieve the same host usage; however, this requires more changes and costs. Furthermore, disposable bioreactors can avoid cumbersome and detrimental silicidation operations when using microcarriers. Disposable benchtop bioreactors radically reduce the routine maintenance of laboratory staff, as did the introduction of disposable shake flasks in mammalian cell culture 20 years ago.
Table 1 compares the glass and disposable reactors and it is expected that the controller life of the disposable bioreactor will increase by 25% over the glass reactor. If a disposable reactor is not used, the number of glass reactors needs to be increased to achieve the same host usage; however, this requires more changes and costs. Furthermore, disposable bioreactors can avoid cumbersome and detrimental silicidation operations when using microcarriers. Disposable benchtop bioreactors radically reduce the routine maintenance of laboratory staff, as did the introduction of disposable shake flasks in mammalian cell culture 20 years ago.
Simulating the design of a mature glass reactor
 Glass bioreactors have been used for decades and have proven to be a reliable scale-up and reduction model for stainless steel and the latest large-scale disposable stirred reactors. The UniVessel® disposable culture vessel simulates the design of a traditional glass benchtop bioreactor to ensure that the newly acquired data is comparable to the data obtained when the glass system was previously used. Each unit is irradiated and sterilized before delivery, so it can be used out of the box. It is equipped with a non-invasive disposable pH and dissolved oxygen (DO) electrode, so conventional electrodes are loaded into the reactor in a laminar flow clean bench before cell culture (Photo 1). Initially, disposable systems faced the challenges of reliability and durability—especially pH-detecting photochemical patch components. But today's significant improvements in chemical and control of disposable patches have ensured reactor reliability and durability (1).
Glass bioreactors have been used for decades and have proven to be a reliable scale-up and reduction model for stainless steel and the latest large-scale disposable stirred reactors. The UniVessel® disposable culture vessel simulates the design of a traditional glass benchtop bioreactor to ensure that the newly acquired data is comparable to the data obtained when the glass system was previously used. Each unit is irradiated and sterilized before delivery, so it can be used out of the box. It is equipped with a non-invasive disposable pH and dissolved oxygen (DO) electrode, so conventional electrodes are loaded into the reactor in a laminar flow clean bench before cell culture (Photo 1). Initially, disposable systems faced the challenges of reliability and durability—especially pH-detecting photochemical patch components. But today's significant improvements in chemical and control of disposable patches have ensured reactor reliability and durability (1).
Easily upgrade existing controllers
UniVessel disposable containers can be easily integrated into new or existing bioreactor controls, reducing the cost of converting from a desktop system to a one-off system. In addition, the pH value can be controlled by integrating a single electrode signal through the UniVessel® connection box. It eliminates the need for traditional electrode installation and eliminates the need for cumbersome and risky operations. In addition, the same optical electrodes and pH and DO measurements were used in the larger Biostat® STR disposable bioreactor. Figures 1 and 2 compare classic and disposable sensors by simulating step changes in pH and DO—showing a good correlation between the probes. Conventional probes can be used in both systems.
Excellent compatibility
Suspension cultures of recombinant CHO cells (usually in serum-free or protein-free conditions) are widely used in the production of modern large-scale monoclonal antibodies (MAbs) and other therapeutic proteins. Since most commercial production development or troubleshooting of new products is based on the experience of a large number of pilot glass reactors in the past, the acquisition of comparative parameters for different cell culture systems is limited.
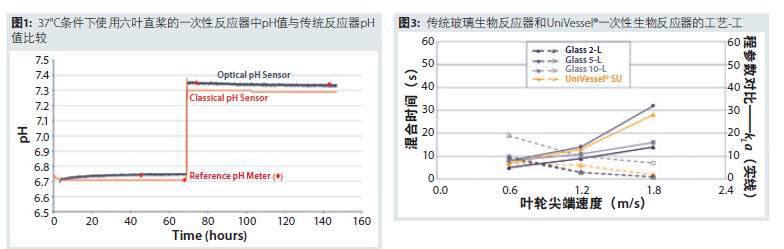
Figure 3 shows the excellent comparability of the most important engineering parameters kLa and mixing time in conventional glass bioreactors and UniVessel® disposable bioreactors at conventional tip speeds (3). In modern monoclonal antibody production, most companies use platform technology and adaptable Chinese hamster ovary (CHO) cell lines to avoid amplification comparability issues. However, when dealing with complex glycosylation-modified recombinant proteins and vaccines, the parameter comparability and scalability of the reactor are extremely important. Especially when using microcarrier culture to produce vaccines, careful consideration must be given to the scalability and comparability of the cell culture systems used (4).
 Download full text view:
Download full text view:
Case Study: Microcarrier-Based Vaccine Process
download immediately
in conclusion
We have demonstrated that the single-use 2-L stirred vessel (UniVessel® SU 2L) has a kLa and mixing time compared to the proven 2-L, 5-L and 10-L stirred glass vessels. Excellent comparability. The team also evaluated the biocompatibility comparability of the adherent cell microcarrier culture process in viral vaccine production. The results show that the disposable 2-L container, the traditional 10-L agitated glass container enlargement/reduction model, the large-scale disposable container (BIOSTAT STR® 50L and 200L) and the stainless steel agitated bioreactor are comparable Cell growth and metabolic properties. Based on these data, GSK has established a new process development platform consisting of 12 parallel 2-L disposable bioreactors that support multivariate experimental design to achieve efficient and rapid optimization of vaccine production processes.
Download the full text now
Porcelain Mortar & Pestle,Mortar Pestles,Medical Mortar Pestle,Porcelain Mortars Spout
Yancheng Rongtai Labware Co.,Ltd , https://www.shtestlab.com