Dezhou City Ducheng Food and Drug Administration to train medical equipment monitoring and testing
The safety monitoring personnel of the Decheng City Food and Drug Administration went to the Decheng Maternal and Child Health Hospital to conduct training on the monitoring of adverse medical events. More than 80 hospitality deans and medical staff attended the training.
The safety monitoring personnel of the Food and Drug Administration of Decheng District of Dezhou City systematically explained the monitoring of medical device adverse events from the aspects of monitoring significance, specific cases, risk signal identification and reporting requirements, and further guided the medical staff to grasp the findings and reports. Effective work measures to alert and control the risk of using medical devices.
After the meeting, the relevant leaders of the hospital indicated that the training enabled medical personnel to have a deeper understanding of the adverse events of medical devices, and the safety monitoring of drug and hospital in hospitals will certainly improve rapidly.
In the next step, the Decheng District Food and Drug Administration Bureau will use the upcoming special rectification of medical institutions to increase the training and supervision of the medical device safety monitoring work in medical institutions to ensure better work this year.
INTENDED USE
The One Step HCG Pregnancy Rapid Test Kit is a rapid
chromatographic immunoassay for the qualitative detection ofhuman chorionic gonadotropin (HCG) in urine to aid in the early
detection of pregnancy.
PRINCIPLE
The test utilizes antibodies including a
monoclonal HCG-β antibody and goat anti-mouse IgG on thenitrocellulose membrane with colloidal gold marked anti-HCG-α
monoclonal antibody as an mark tracer. The reagent is used to
detect the HCG in urine according to the principle of double
antibody sandwich method and gold immunochromatography
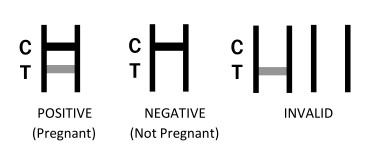
assay
There is a control line (C) controlling the reaction process shown
on the coated film. Based on test line`s (T) appearance to
determine whether the tested sample contains HCG (Human
Chorionic Gonadotrophin) or not.
MAIN COMPONENTS
Basic components: Sample pad, colloidal gold marked pad,
nitrocellulose membrane, absorbent paper and PVC board.

SAMPLE REQUIREMENTS
A urine specimen must be collected in a clean and dry container. A
first morning urine specimen is preferred since it generally
contains the highest concentration of HCG, however, urine
specimens collected at any time of the day may be used. Urine
specimens may be stored at 2-8℃ for up to 48 hours prior to assay.
For long-term storage, specimens may be frozen and stored below
-20℃, the frozen specimens should be fully melted and restore to
room temperature and shake before testing .Urine specimens
exhibiting visible precipitates should be centrifuged, filtered, or
allowed to settle to obtain a clear specimen for testing.
Pregnancy Test Kit,HCG Test Strips,HCG Urine Test Kit,HCG Pregnancy Rapid Test Kit
Changchun ZYF science and technology CO.,LTD , https://www.zyf-medical.com