Postpartum depression new drug brexanolone submitted to NDA is expected to fill the treatment gap
Postpartum depression new drug brexanolone submitted to NDA is expected to fill the treatment gap
April 24, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Sage Therapeutics announced today that it has submitted a new drug application (NDA) to the US FDA for the treatment of postpartum depression (PPD) with brexanolone (SAGE-547) intravenously.

Postpartum depression is a common birth-related complication. It is a serious emotional disorder, including depression, temper, loss of appetite, fatigue, insomnia, guilt and a series of symptoms that prevent women from living properly. It is also the leading cause of suicide in postpartum women. However, there is currently no FDA-approved postpartum depression therapy. There are also important medical needs in this area that are not being met.
Brexanolone is an allosteric modulator of the innovative GABAA (gamma-Aminobutyric acid) receptor developed by Sage. It regulates the function of GABAA receptors located in and outside the synapses. GABAA receptors and NMDA (N-methyl-D-aspartate) receptors act to inhibit and stimulate brain neurons to produce nerve impulses, respectively. The imbalance between the two receptor activities is responsible for a variety of mental illnesses including depression. Brexanolone efficiently and safely restores the balance between GABAA receptor and NMDA receptor activity. It has received the US FDA's breakthrough therapy approval and the European Medicines Agency (EMA) Priority Medicine (PRIME) for the treatment of postpartum depression.
The new drug application submitted this time was supported by the Hummingbird project data. The clinical program included three multicenter, randomized, double-blind, parallel-group, placebo-controlled trials (Study 202A, Study 202B, and Study 202C). These studies evaluated the safety and efficacy of brexanolone in women with moderate or severe PPD between the ages of 18 and 45 and within 6 months of postpartum.
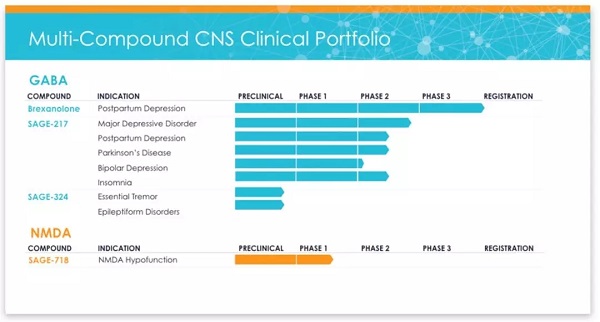
â–² Sage Therapeutics R & D pipeline (Source: Sage Therapeutics official website)
In Phase 2 trial 202A, 21 women with severe PPD were randomized to brexanolone or placebo. These patients had a Hamilton Depression Rating Scale (HAM-D) score of more than 28 before treatment. Studies showed that patients receiving brexanolone had a significant difference in HAM-D reduction compared with placebo (-19.37 vs. -8.22, p=0.006). In the 30-day review after treatment, the HAM-D decline was still significantly higher in the brexanolone group than in the placebo group (-20.77 vs-8.84, p=0.010). Phase 3 clinical trials of 202B and 202C showed that brexanolone reached the primary endpoint in both trials. The 60-hour HAM-D score was significantly lower than baseline in the placebo group (Study 202B: 90 μg/kg/h dose p = 0.0242, 60 μg/kg/h dose p = 0.0011; study 202C, 90 μg/ Kg/h dose p = 0.0160). In both trials, patients receiving brexanolone had a 14-20 reduction in HAM-D total score over 60 hours and were maintained for 30 days. In addition, brexanolone showed good safety and tolerability in the trial, no patients died due to treatment, or due to serious side effects and discontinuation of the trial.
“Postpartum depression is often thought of as a disease that the mother has experienced, but it affects children and family members in the short and long term and should not be ignored by new mothers or people around them. In these studies, brexanolone is The study provided in-depth and long-lasting results that may change the perception of medical institutions treating the disease.†202B and 202C, the main investigator, the UNC Center for Women's Mood Disorders Dr. Samantha Meltzer-Brody, Director and Associate Professor of the Perinatal Psychiatry Program, said.
We expect this new drug to be reviewed and marketed as soon as possible, so that mothers who experience postpartum depression can easily overcome the difficulties.
Reference materials:
[1] Sage Therapeutics Submits New Drug Application to US FDA for Intravenous Brexanolone in the Treatment of Postpartum Depression
[2] WuXi PharmaTech - Phase 3 Success! New drugs for postpartum depression are expected to fill the treatment gap
Calcium Acetate Monohydrate,Calcium Acetate,Calcium Acetate Powder,Calcium Ethanoic Acid Salt
Wuxi Yangshan Biochemical Co.,Ltd. , https://www.yangshanchem.com