What are the latest liver cancer research and development pipelines in February 2018?
What are the latest liver cancer research and development pipelines in February 2018?
March 23, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Primary liver cancer is the sixth most common cancer in the world (6%) and the second most common cancer. It is estimated that 750,000 people die of liver cancer every year worldwide. The main cause of liver cancer is cirrhosis caused by hepatitis B, hepatitis C or alcohol. The most common types of liver cancer are hepatocellular carcinoma (HCC) and cholangiocarcinoma, which account for about 80% of liver cancer. The area of ​​the disease varies widely, with approximately 80% of new cases occurring in Asia, including China and Japan.
Liver cancer is a serious and high-risk cancer, but treatment options are very limited. Most systemic drugs are not effective in treating liver cancer. In November 2007, the FDA approved sorafenib for the treatment of advanced hepatocellular carcinoma. Sorafenib is a targeted drug that blocks cell proliferation and cell growth and provides survival benefits for patients with advanced liver cancer. The systemic treatment currently approved for first-line therapy for liver cancer is very limited and highlights this unmet medical need.
â–²Little knowledge of liver disease (Source: 123RF)
This article takes stock of the latest clinical development pipeline for liver cancer in February 2018. We calculated clinical trial data for clinical, phase 2, and phase 3 liver cancer drugs enrolled at clinicaltrials.gov. Included in the statistics are trials that are being recruited, recruited, and issued recruitment notices, and do not include trials that have been completed, terminated, unknown, withdrawn, and suspended.
New drug pipeline inventory in the clinical stage of liver cancer
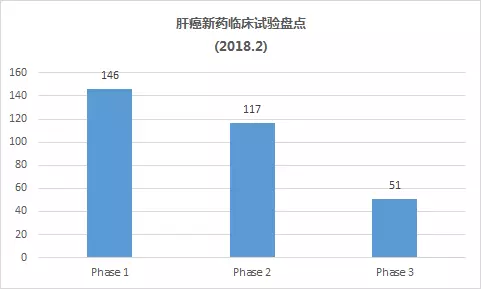
As of February 28, 2018, there were 314 clinical trials of new drugs in the field of liver cancer, and early clinical research was dominant. These include: 146 trials in Phase 1, including 109 small molecule drugs and 37 biopharmaceuticals; 117 trials in Phase 2, including 100 small molecule drugs and 17 biopharmaceuticals; 51 trials, including 45 small molecule drugs and 6 biological drugs (note: some drugs may be tested simultaneously).
Clinical trials of new liver cancer drugs are concentrated in the United States and Asia. According to the statistics of the research institutes of clinical trials, the top 5 countries or regions are: 141 trials in the United States, 82 in China, 35 in Taiwan, 33 in South Korea, and 32 in France (Note: There may be multi-center clinical trials ).
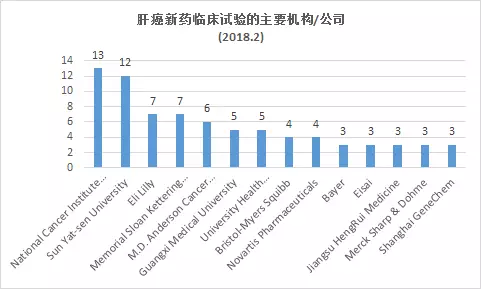
The top five research institutions for conducting clinical trials of new liver cancer drugs are: National Cancer Institute (NCI) 13 trials, Sun Yat-sen University 12 trials, Memorial Sloan Kettering Cancer Center 7 and MD Anderson Cancer Center 6 Item, 5 Guangxi Medical University.
The top 5 companies are: Lilly 7 trials, Bristol-Myers Squibb 4, Novartis 4, Bayer, Eisai, Merck, Jiangsu Hengrui and Shanghai Jikai genes are each three trials.
New clinical trial of new liver cancer drugs in February
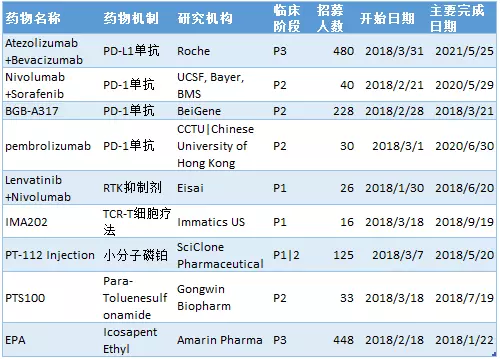
In February 2018, nine new clinical trials of liver cancer were added. Two out of phase 1 trials recruited 26 subjects, one 1/2 phase trial enrolled 125 subjects, four phase 2 trials enrolled an average of 82 subjects, and two phase 3 trials enrolled an average of 464 patients.
â–²A new clinical study of new liver cancer drugs in February 2018
It is worth noting that four (44%) new clinical trials of new liver cancer trials were conducted on immunological checkpoint inhibitors, including PD-1 monoclonal antibody and PD-L1 monoclonal antibody. One is TCR-T cell therapy. The following is a new clinical trial of liver cancer and an introduction of new drugs in February.
New drug name: BGB-A317 (tislelizumab)
Drug mechanism: PD-1 monoclonal antibody
Research institute: Baiji Shenzhou (BeiGene )
Clinical Phase: 2
Tislelizumab is an immunological checkpoint inhibitor PD-1 monoclonal antibody developed by Baekje Shenzhou and is currently undergoing critical trial evaluation in four different indications. This is a phase 2 open-label, multi-center clinical trial designed to study the efficacy, safety, and pharmacokinetics of anti-PD-1 monoclonal antibody BGB-A317 in patients with previously untreated unresectable hepatocellular carcinoma. The trial plan recruited 228 subjects. The expected start date for the study was February 28, 2018, and the expected major completion date was March 2021.
It is worth noting that Baekje Shenzhou has launched a global phase 3 clinical trial of Tislelizumab to evaluate the safety and efficacy of this drug in patients with previously untreated advanced hepatocellular carcinoma (HCC). Sorafenib, the standard treatment for advanced global liver cancer.

New drug name: Atezolizumab
Drug mechanism: PD-L1 monoclonal antibody
Research institutions: Roche (Roche)
Clinical Phase: 3
In May 2016, the US FDA accelerated the approval of Atezolizumab for the treatment of locally advanced or metastatic urothelial carcinoma, and was the first approved PD-L1 drug. This new clinical study will evaluate the efficacy of atezolizumab in combination with bevacizumab in patients with locally advanced or metastatic hepatocellular carcinoma (HCC) and systemic therapy compared with sorafenib (Sorafenib). And security. The trial plan recruited 480 subjects. The expected study start date is March 31, 2018. The expected major completion date is May 25, 2021.

New drug name: Sorafenib+Nivolumab
Drug mechanism: PD-1 monoclonal antibody
Research institute: Bayer, Bristol-Myers Squibb
Clinical Phase: 2
This study was designed to determine the optimal tolerated dose and safety of Sorafenib in combination with immunological checkpoint inhibitor nivolumab in patients with hepatocellular carcinoma (primary liver cancer) who cannot be surgically resected. Sorafenib blocks the growth of cancer cells by blocking some of the enzymes needed for cell growth. Immunological checkpoint inhibitors, such as nivolumab, can help the immune system suppress cancer. The trial plan recruited 40 subjects. The expected study start date is February 21, 2018. The expected major completion date is December 31, 2020.

New drug name: Pembrolizumab
Drug mechanism: PD-1 monoclonal antibody
Research institute: The Chinese University of Hong Kong
Clinical Phase: 2
Pembrolizumab is a PD-1 monoclonal antibody from Merck (MSD) and is marketed in the United States under the trade name Keytruda. This is a one-arm phase 2 clinical trial of pembrolizumab for patients with hepatitis B virus-associated hepatocellular carcinoma, with parallel studies of baseline and immune environment changes. The primary objective of this trial was to investigate the efficacy and safety of pembrolizumab in patients with HBV-associated HCC, and to study the continuous changes in the expression of immune-related genomic RNA in biopsies after treatment. The secondary objective was to study a series of changes in cytokine profiles between pre- and post-treatment samples, PD-L1 immunohistochemistry (IHC) expression, and tumor infiltrating lymphocytes. The purpose of this trial was to assess the likelihood of a treatment response predicted by changes in RNA expression measured by immunologically related gene sequencing or PD-L1 / 2 IHC. The trial plan recruited 30 subjects. The expected study start date is March 1, 2018, and the expected major completion date is June 30, 2020.

New drug name: Lenvatinib ( Lenvaninib )
Drug mechanism: receptor tyrosine kinase (RTK) inhibitor
Research institutions: Eisai (Eisai)
Clinical phases: 1
Lenvatini (Lenvarinib) is an oral multi-receptor tyrosine kinase (RTK) inhibitor developed by Eisai, which selectively inhibits VEGF receptor, FGF receptor, and RTK pathway. Countries including Japan and the United States are approved for the treatment of refractory thyroid cancer, and second-line treatment of advanced renal cell carcinoma with everolimus. In November 2017, lenvatini submitted a marketing application for the treatment of advanced liver cancer in China, which is expected to be approved within the year. The US FDA has also accepted a new drug application for lenvatinib for first-line treatment of hepatocellular carcinoma (HCC). In addition, lenvatini is also undergoing clinical research on a variety of solid tumors, including first-line therapy for renal cell tumors, non-small cell lung cancer, and cholangiocarcinoma.
In February of this year, Eisai submitted a phase 1 clinical study evaluating the tolerance and safety of lenvatinib in combination with nivolumab in the treatment of hepatocellular carcinoma (HCC). The trial plan recruited 26 subjects. The expected study start date is January 30, 2018. The expected major completion date is June 2020.

New drug name: IMA202
Drug Mechanism: TCR-T Cell Therapy
Research institute: Immatics
Clinical phases: 1
IMA202 is a research-based immunotherapy that uses Immatics' proprietary ACTengine® method to genetically engineer patients' own T cells to express exogenous TCR. The goal is to redirect and activate cells to treat solid tumors. Immatics is a clinical stage biopharmaceutical company dedicated to the discovery and development of T cell reversal immunotherapy for the treatment of cancer.
The aim of this study was to determine the safety and tolerability of IMA202 products in certain solid tumor patients, including squamous cell non-small cell lung cancer or hepatocellular carcinoma. In this trial, IMA_Detect was developed as a companion diagnostic to help select patients with relapsed and/or refractory solid cancer who may be eligible to participate in clinical trials. The trial plan recruited 16 subjects. The expected start date for the study was March 2018, and the expected major completion date was September 2019.

New drug name: PT-112
Drug mechanism: small molecule phosphoplatin
Research Institute: SciClone Pharmaceuticals
Clinical Phase: 2
This is a multicenter, open-label, phase 1 clinical trial of a phase 1 dose escalation study in patients with advanced solid tumors, including hepatocellular carcinoma, gastric cancer, colorectal cancer, non-small cell lung cancer, head and neck cancer, and breast cancer. . After obtaining sufficient data during the dose confirmation phase, Phase 2 trials were performed in patients with hepatocellular carcinoma (HCC).
Principal Investigator Dr. Jin Li from Shanghai Oriental Hospital. The trial plan recruited 125 subjects. The expected study start date is March 7, 2018. The expected major completion date is May 2020.
DC Bead® (Embolisation bead), a novel product of Saisheng Pharmaceutical's drug-loaded microspheres for interventional treatment of primary liver cancer, has been approved in China. PT-112 injection is an intravenous injection of small molecule phosphoplatin for the treatment of solid tumors. It has also been submitted for clinical application for the first time in China.

Note: The title map source 123RF
Reference materials:
[1] ClinicalTrials.gov
[2] Company's official website
PET Anti Slip Tape
Our PET anti slip tape is made of premium PET material and selected aluminum oxide grit, coated with special upgraded solvent acrylic adhesive. It has super strong durability, long lasting high adhesion, uniform sand surface, good waterproof ability. It can be used instead of 3M 600 series, function and quality are similar, but the price is much more economical. Service life of our PET anti slip tape is 2 years. It is perfect to be used for flat surfaces, steps, stairways, entrances, ramps, ladders, lawn equipment, snowmobiles, scooters, construction machinery and vehicles, other machinery and vehicles
It has different colors, such as black, transparent, grey, yellow, yellow/black, etc. Other colors also can be customized. The width ranges from 10mm to 1070mm, the length can be 2meters to 120.2meters.


PET anti slip tape, anti slip grip tape, 3M anti slip tape, safety-walk slip resistant tape, safety walk anti slip tape
Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.jerrytape.com