Laser fluorescence promotes the development of sequencing technology
Laser fluorescence promotes the development of sequencing technology
June 23, 2015 Source: Bio Valley
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];DNA sequencing, from any angle, represents the most dynamic area of ​​bioassay. Although it has been 25 years of development, sequencing technology has not been combined into a single basic method, but has developed an increasingly diverse and diverse technology (see "Expression Spectrum and Integrated Sequencing" on page 26). However, laser-excited fluorescence technology remains the most popular detection method. In fact, laser fluorescence technology plays a key role in the development of gene sequencing, and rapid changes in the sequencing environment are driving important product trends.
Speaking from the Sanger law
The first successful method for determining a DNA sequence was the chain termination method, also known as the Sanger method. This method uses a polymerase to replicate single-stranded DNA in four different deoxyribonucleoside bases and a low concentration of chain-terminated nucleotides; the chain-terminating nucleotides have been chemically modified so that the polymerase can absorb One of these nucleotides, but its structure will prevent further synthesis. As a result, fragments of different lengths will be produced from the original primer sequences, from only a few bases to the same length as the original single strand. After radiolabeling the terminating nucleotide and dispersing the replica in a silica gel plate, the sequence can be rendered on the exposed photo substrate with four unique stripes (adenine [A], cytosine [C], guanine [G] "Barcode" of thymine [T].
The method was automated by first-generation instruments using a fluorescently-labeled chain-terminated nucleotide (four bases labeled with four different fluorescent colors) to distinguish the replicated fragments by capillary electrophoresis (CE). Length, followed by laser fluorescence (usually 488 nm) to identify A, C, G, T (see Figure 1).
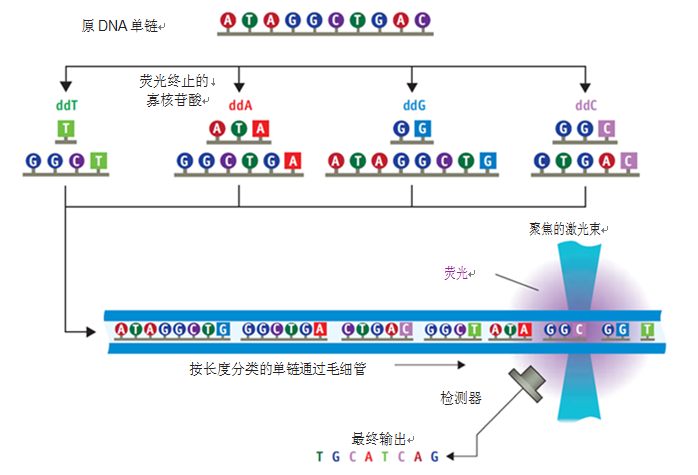
Figure 1. The initial Sanger sequencing method begins at the end of the polymerization reaction, is labeled with radionucleotides, and is then electrophoretically differentiated on a gel spacer. The method was subsequently switched to fluorescent nucleotide labeling, capillary electrophoresis discrimination and laser detection to achieve the first generation of automated sequencing.
Only a few hundred bases can be sequenced per round. Therefore, the entire human genome is shared by multiple laboratories, each running multiple sequencers at a total cost of approximately $3 billion for up to 10 years.
Second generation: massively parallel computing
Incredibly, the cost of sequencing the entire human genome has been reduced by nearly seven orders of magnitude, and the cost of sequencing each complete human genome is now less than $1,000. Developers of third-generation instruments have gone even further and can continue to reduce costs to one-tenth.
The massively parallel computing used in second-generation sequencing (NGS) instruments is an important step in driving speed and cost innovation. The two most popular second-generation sequencing methods have been validated by Illumina (San Diego, CA) and Life Technologies (Carlsbad, CA). Both of these methods first cut the target DNA into easy-to-handle single strands, each of which typically contains hundreds of bases. Subsequently, these single strands are arranged into a non-overlapping array and amplified in situ, ie, by polymerase chain reaction (PCR) or cloning to form small clusters, each cluster containing only one single strand and by this species Many identical copies of a single chain. Subsequent fluorination tracking is performed with a megapixel sensor, so that up to tens of thousands or even hundreds of thousands of clusters of laser fluorescence can be analyzed simultaneously. Life Technologies uses ligation (scission) sequencing, while Illumina uses synthetic sequencing (SBS). The latter has become a market-leading brand, thanks in part to the ultra-high throughput of its flagship instrument, with a daily sequencing of 600 billion bases, making it ideal for whole-genome sequencing and other similar applications.
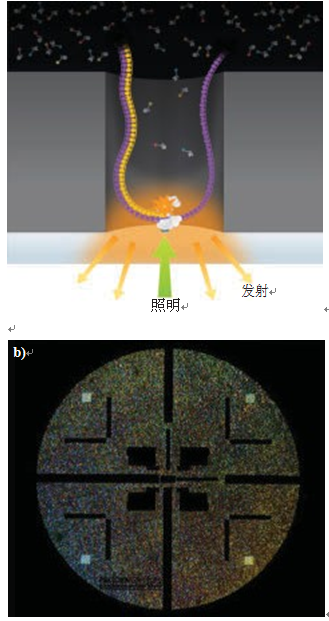
Figure 2. Pacific Biosciences RS II Sequencer. Each zero mode waveguide (ZMW) contains a single polymerase (Figure a). By directing the laser separation to the many diffraction-limited spots of each ZMW, up to 150,000 ZMWs can be simultaneously simulated and monitored on a single SMRT chip (Figure b). (Image courtesy of Pacific Biosciences)
Sequencing of both methods was performed on a glass plate or flow cell excited by a laser. Each nucleotide (A, C, G, and T) has a unique fluorescence emission profile. Thus, each unique cluster will flash in a particular wavelength mode, ie, appear in a different color at its imaging location to correspond to the next base that will be cut (Life Technologies) or merged (Illumina). Combined with the use of dichroic filters and low-noise digital camera detection, the bases of millions of cluster sequences can be read one by one in each chemical cycle. The computer then compares all sequences from these randomly aligned clusters and compares them to known/expected genomic sequences to form the complete uncut DNA complete sequence.
Genetics / DNA Sequencing (continued)

Figure 3. Instrument manufacturers often look for integrated lasers and beam delivery solutions, and sometimes expect products to offer multiple laser wavelengths. Coherent's Galaxy solution provides off-the-shelf technology that combines the output of multiple lasers into a single output fiber using eight wavelength-specific plug-and-play inputs.
Third Generation:
Technical diversity
Looking ahead, several "third generation" sequencing methods will appear on multiple commercial development platforms, including new systems based on electrochemical and semiconductor detection. However, for some reason, laser fluorescence will continue to be the mainstream method: this method is widely accepted and is the most versatile method for wet chemical and biochemical real-time sampling, providing unparalleled spatial resolution and high levels. The sensitivity is even up to the single molecule level.
The goal of most third-generation methods is to reduce costs and even increase overall throughput. The reason why we emphasize the "whole" flux is not only the speed of determining the flux, but also the number of bases per round, the number of single strands per round, and the length of single strands. The second-generation sequencing method has the characteristics of large read length and short length, while in the third generation method, the number is relatively small and the length is very long. This simplifies the problem of single-strand overlap of oligonucleotides present in the software when assembling a complete target sequence, thereby reducing the necessary computer time and the amount of oversampling (sampling depth).
Oversampling is necessary because second generation sequencing and some third generation methods often have higher error rates. Unlike the Sanger improvement with nearly 100% accuracy, the error rate for some technologies (per single read event) can reach 15% or higher. However, the occurrence of errors is a random event; in essence, the oversampling used by these methods still provides 100% accuracy. For example, even if each base of the read length is only 80% accurate, then 15 to 30 times the oversampling amount can achieve a very small error rate.
Pacific Biosciences (Menlo Park, Calif.) has developed a very interesting method in which an innovative optical component is used. The PacBio RS II system uses a zero mode waveguide (ZMW), an optical waveguide that is much smaller in diameter than the wavelength of the light. On a single molecule real time (SMRT) chip, a circular hole (about 70 nm in diameter) was formed in an aluminum film (100 nm thick) deposited on a transparent silica substrate to generate ZMW. There is a single polymerase at the bottom of each waveguide. Laser illumination penetrates the silica gel and enters each waveguide to a depth of only 30 nm, so the millisecond pulse of fluorescence is efficiently absorbed only when nucleotides are added to the DNA. Importantly, the company claims a single continuous read length of up to 40,000 bases. The utility of sequencing tools is determined by the degree of parallelism, and the degree of parallelism can be achieved by a simple experiment of amplification, and this depends to a large extent on the available laser technology. The PacBio RS II instrument monitors up to 150,000 ZMW in real time by separating the laser into a number of diffraction-limited spots pointing to each ZMW (see Figure 2). According to Paul Lundquist, senior manager of optical engineering and instrument design at Pacific Biosciences, the company's Genesis technology is well suited for this application. "It is not difficult to generate a diffraction-limited spot from a laser beam, but an optical system that meets this level of beam separation and precise positioning requirements must have excellent beam quality and wavelength stability. It is because of these high performance requirements. And the scalable power platform, Genesis performed very well."
Expression profiling and comprehensive sequencing
The diversification of the application market is one of the reasons for the increasing diversity of sequencing methods. The clinical application area is relatively small in the DNA sequencing market, but it is expected to see a nearly doubled increase in growth by 2019 (exclusive survey results). In addition, only some research and clinical applications require reading gigabases at the single-base resolution level, such as the complete sequencing of the first human genome. There are some areas of application (for example, finding point mutations associated with certain cancers or developing transgenic crops) that require a single-base resolution of short fragments.
For the field of expression profiling applications, the goal is to find out if certain sequences or genes are present and/or their positions, rather than reading each base. The method of BioNano Genomics (San Diego, Calif.) is very interesting, combining novel microfluidics with laser-excited fluorescence. This method inserts a fluorescently labeled single-stranded DNA strand into the parsed double helix DNA. Each single-stranded DNA strand is inserted into it only if it has a pair that is fully complementary to it in the double-stranded DNA. The DNA is then passed through a narrow microfluidic channel to extend the DNA tertiary structure to form a long string of shapes. The laser stream is then used to traverse the DNA stream and the inserted DNA fragments are read as if they were a color barcode.
At least in the short term, these third-generation technologies will compete with second-generation sequencing methods, so it is necessary to focus on several interesting advances in the field of optoelectronics as the mainstream second-generation sequencing technology. One of the advances was the attachment of oligonucleotide single strands to miniature glass beads (3 μm) rather than randomly placed on a surface. These single chains are bridge-amplified and self-assembled in dense pits or wells at lower levels of the flow cell. This ensures the highest density while avoiding clusters or glass beads overlapping. In addition, in the microelectromechanical processing system (MEMS) technology, a set of near parabolic reflectors are arranged on the outer surface of the lower surface of the flow cell, which can concentrate the laser on each glass bead and absorb the epifluorescence efficiently. Subsequently, the laser is efficiently concentrated into the concentrator using a microlens. Another advancement in the field of fluorine chemistry simplifies the optical component system and reduces the total amount of image demand. Specifically, some systems use double-stained sequencing chemistry and dual-channel detection, where "C" is only red, "T" is only green, "A" is red-green, and "G" is dark (non-red and green) .
Laser performance and integration
These impressive innovations in chemistry, nanophotonics and microfluidics drive the four main trends in lasers: diversity, reliability, integration and collaboration.
Laser diversity. The dynamic nature of sequencing and the nascent state of the end market (currently the market share of clinical applications is very limited) makes the future difficult to predict. The market requires only a few wavelengths (488 nm, 514 nm, and 532 nm, and red at 640 nm or 660 nm), but the need for other parameters of the laser is diverse. This means that in order to provide the widest range of standard laser options to support R&D, more than one core technology (such as diodes, optically pumped semiconductor lasers [OPSL]) is often required. This also means that in order to support an optimized, larger volume instrument, it should be possible to manufacture a cost-effective custom laser.
For example, coherent companies offer laser power ranging from a few milliwatts to 10 watts. why? For applications where the DNA single strand is highly amplified and spatially highly concentrated, the desired fluorescent signal is stronger and the laser power is lower. However, if these sampling points are spread over large flow cells or chutes that require large areas of illumination, the power requirements are increased. For applications where sequencing is single-strand and where the signal is relatively low, power becomes very important, especially in applications where large-scale parallel computing is often performed. Another factor in the power equation is damage; the laser must not cause any functional damage to the enzyme used in sequencing or to the DNA itself. Because shorter wavelengths tend to cause more damage, less than a few milliwatts of power for shorter wavelengths (such as 488 nm).
Laser reliability. While most commercial laser applications have reliability requirements, the issues here are not just the costs of unplanned downtime to the original equipment manufacturer of the instrument and its end users. Certain sequencing technologies, especially for whole-genome applications, require continuous operation for several days.
If the laser has a performance failure or a temporary performance deviation, it will drag the entire data set, resulting in no value. Therefore, the market is very conservative and will not be interested in unproven laser technology.
Laser integration. For most sequencing technologies, the exclusive patented "magic features" and consumer-led profits, mainly from patented chemical and microfluidic technologies, have resulted in relatively few innovations in laser usage. In addition, most of these companies have no investment or expertise in laser generation and transmission/modification. So in most cases, what they are looking for is an integrated optoelectronic engine rather than a standard laser. The number of instrument developers who need plug-and-play simple smart lasers is extremely limited during R&D and product test board experiments. But they often need off-the-shelf modular solutions to achieve plug-and-play synthesis of multiple wavelengths in fiber-optic transmissions (single-mode and multi-mode) and beam shaping using refractive and diffractive optics (see Figure 3). . In some cases, they require the elimination of the coherence of the laser in the engine to avoid spotting and ensure consistent, stable illumination.
Cooperation. Because of these market characteristics, instrument developers and manufacturers often want laser suppliers to be subcontractors rather than component suppliers. This type of partnership requires the laser supplier's ability to manufacture systems that meet certain output specifications, as well as systems that are dedicated to printing, but the latter are less common. In addition to this expertise, as third-generation sequencing technologies have been successfully commercialized and are beginning to compete in a fast-growing market, instrument companies also want laser subcontractors that can cope with unpredictable growth patterns. .
Increase value
Although technology has made great strides, sequencing is still an active early market with an incalculable range of technology diversification possibilities. More importantly, the laser is used as a reliable component rather than an innovative solution. This requires laser manufacturers to produce a wide range of off-the-shelf lasers and beam-transmitting/beam-synthesizing products, and the ability to provide a wide-ranging, customized system to support the industry.
Yogurt Capsules,Probiotics Capsule,Probiotics Supplement,Prebiotic And Probiotic Capsules
Jiangsu Biodep Biotechnology Co. ,Ltd. , https://www.mbioda.com